Calorimetry with Gas Capture
VIDEO LINKS:
Consider before watching the videos:
How would you capture the gas from a reaction?Lab Experiments (Write protocols based on the videos then complete your protocols as you perform experiments in class):
Data analysis and calculations (Use these if you need help with your data and calculations)
Experimental Protocol
(Analysis) Watch the experiment videos. Take notes on the protocols. Stop the videos and re-watch as necessary to acquire details of the procedure. Write out the protocol for each part of the experiment. (It can be written in sequential steps. Complete sentences are not necessary.) This is the protocol you follow, so be detailed.
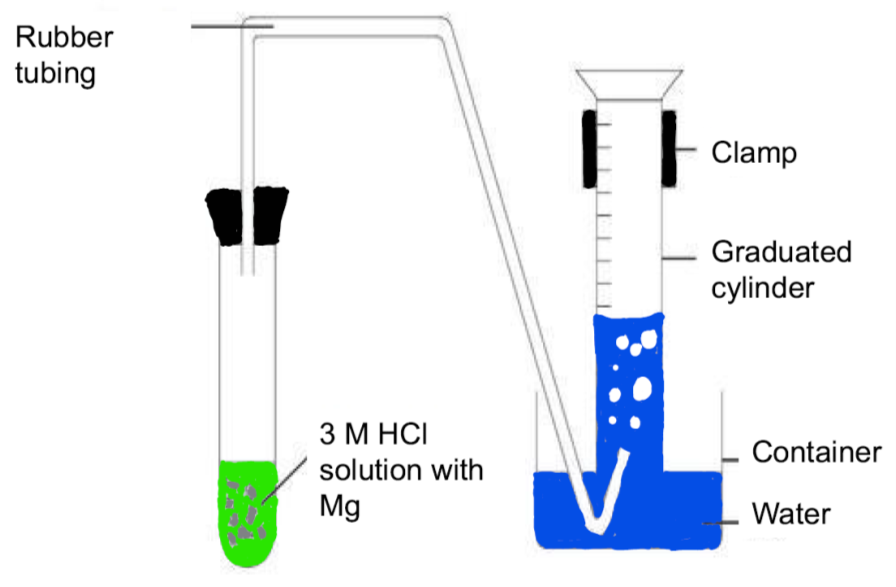

Chemical Table
(Representation) Prepare your chemical table including the materials you use in the experiment. Here is a general template that you may use.
| Chemical Name | Chemical Formula | Molar Mass (g/mol) | Hazards | reference | PPE |
| Sodium Chloride | NaCl | 58.5 | Skin irritation | https://fscimage.fishersci.com/msds/21105.htm | |
Equipment Table
(Analysis) Identify the equipment (type AND size) needed for the experiment and include the name and an image (picture) of each. Be sure to describe the equipment, how to use it, and why it is suitable for this use.
| Equipment Name | Equipment Picture or Description | Intended Purpose |
Data Collection
(Acquiring competencies) Following your detailed protocol, perform all the experiments. Record your observations and take pictures of your key steps in the process. Your observations and images need to be incorporated in your data section and this section should be as detailed as possible as you will use this information to complete your discussion.
Data Processing
- (Representation) Write the balanced chemical equation for the reaction between the magnesium strips and the hydrochloric acid solution.
- (Interpretation) Describe the meaning of the chemical equation on the microscopic (atoms/molecules/formula units) and macroscopic scale (moles).
- (Analysis) Classify the reaction between Mg and HCl, (Hint: it could be multiple types) and justify your choice.
- (Manipulation) Calculate the number of moles of hydrogen gas expected in each experiment if all the Mg ribbon is consumed.
- (Manipulation) Calculate the number of moles of hydrogen gas expected in each experiment if all the HCl is consumed.
- (Analysis) Determine the limiting reactant in the reaction of Mg ribbon and HCl.
- (Manipulation) Calculate the actual moles of hydrogen gas produced using the collected volume of gas in experiment 1. (HINT: the gas was collected over water.)
- (Manipulation) Calculate the actual moles of hydrogen gas produced using the collected volume of gas in experiment 2. (HINT: the gas was collected over water.)
- (Manipulation) Calculate the percent yield of the reaction in experiment 1.
- (Manipulation) Calculate the % yield of the reaction in experiment
- (Manipulation) Calculate the heat absorbed by the water.
- (Analysis) Identify the amount of heat given off by the reaction of the metal with the HCl.
- (Manipulation) Calculate your experimental enthalpy change for the reaction of magnesium with the hydrochloric acid solution.
- (Existing knowledge, research and views) List the standard enthalpies of formation for Mg, HCl, MgCl2 and H2
- (Manipulation) Calculate the standard enthalpy change for reaction using the standard enthalpies of formation.
- (Manipulation) Determine the % error of your experimental value for the enthalpy change for reaction using the calculated enthalpy change from enthalpies of formation as the correct value.
- (Existing knowledge, research, and views) Provide at least 3 additional examples for gas forming reactions and provide a reference for your source.
- (Existing knowledge, research, and views) Define ideal gases in your own words and describe the conditions under which a gas will behave as an ideal gas. Provide a reference for your source.
- (Existing knowledge, research, and views) Identify the position of magnesium and hydrogen on the activity series and provide a reference for the activity series that you used to establish those positions.
- (Analysis) Using the positions of the magnesium and hydrogen on the activity series, predict if the reaction between the magnesium ribbon and the hydrochloric acid solution should proceed.
- (Analysis) Compare the observed reaction between the magnesium ribbon and the hydrochloric acid solution to your earlier prediction about the reaction based on their positions in the activity series. Comment on whether the observations are consistent with your expectations.
- (Assumptions and limitations) Identify at least one assumption that you made about H2 being suitable for this experiment. (Hint: consider polarity and solubility in water.) If this assumption is not valid, describe how it impacts the volume of the gas collected in the experiment.
- (Analysis) Propose at least two other gases that would be similarly suited for such an experiment and two gases that would not be. Support your choices with relevant information about the proposed gases.
- (Analysis) Identify at least one of the 12 principles of green chemistry applied in this experiment. Justify your selection. 12 Principles of Green Chemistry
- (Assumptions and Analysis) Fill in the following table using the observations and data from your experiments.
| Assumptions made | Testing the assumption | If assumptions are wrong ... |
| All the acid will be consumed in the reaction. | ||
Summary
The summary has two parts: an abstract and an experimental protocol reflecting the experiment that you actually performed. The abstract and experimental protocol should both be between 100-250 words, size 12 Arial font each. There may be multiple experimental protocols for one experiment.Part 1.
Title of Experiment: Performed tasks and applied techniques to accomplish the goal of the experiment and a description of the system under study (the title you are given for the lab is insufficient). Ex. Calorimetry of a gas forming reaction with 3M HCl and magnesium ribbonAbstract
(This is a highlight of what you learned from performing the experiment and processing your collected data. Below is a template and an example of what you are expected to write.)
In this lab experiment we investigated [purpose of your experiment] using [list the materials you worked with] and we found that [present the highlights of your results (include numbers)]. The results [present significant results]. The collected data compares to [draw conclusion about your results compared to other sources (this could be another group or the literature value)]. The [add type of measurement individually] measurement was collected using [describe instrument by sensitivity].
Think through this before writing:
- Purpose: What were you trying to do?
- Materials used: What chemicals and materials did you work with?
- Method: What instruments were used to collect measurements and how were the measurements collected?
- Results: What did you find out about the objects under study?
- Significance: What did you learn about the precision and accuracy of your techniques? How do your determined values compare to reported values?